|
|
Original Article
| ||||||
| Microbial quality of herbal powders in Ghana | ||||||
| Eric Agyeman-Duah1, Esi Yaaba Quaidoo2, Felix Charles Mills-Robertson3 | ||||||
|
1Teaching/Research Assistant, Department of Pathology, Kwame Nkrumah University of Science and Technology/Komfo Anokye Teaching Hospital, Kumasi, Ghana
2MSc, Department of Nutrition, University of Ghana, Accra, Ghana 3Lecturer, Department of Biochemistry, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Agyeman-Duah E, Quaidoo EY, Mills-Robertson FC. Microbial quality of herbal powders in Ghana. Edorium J Microbiol 2017;3:10–17. |
|
Abstract
|
|
Aims:
A large percentage of the Ghanaian population relies on herbal medicine solely to meet their basic healthcare needs. However, medicinal plant materials normally carry a large number of microbes and the presence of these microbial contaminants has the potential to cause disease in humans when consumed. This study evaluates the microbial load and characterizes pathogens in three of the most patronized herbal powders in the Kumasi metropolitan area.
| |
|
Keywords:
Bacteria, Health risk, Herbal powder, Microbial load, Yeast and Mold
| |
|
Introduction
| ||||||
|
Herbalism is prehistoric. For example, indigenous physicians in ancient times left texts of medicinal recipes and those who did not leave it in text passed on their information on medicinal herbs to their progeny by having them participate in preparing the medicament [1]. There is an extensive list of reasons why herbal medicine is so important and it is evident since about 80% of the world’s population depends on herbal products for their primary healthcare [2]. It has been established that herbal medicine is used three times as much as conventional medicine worldwide, and have managed to stand the test of time by passing down from generation to generation [3]. Statistics have shown that about 65% of Ghanaians rely on herbal medicine to meet their basic healthcare requirements [4]. In a recent African study for the Roll Back Malaria Initiative, it was discovered that countries like Ghana and Nigeria used herbal therapies as home remedies (first point of call) in nearly two-thirds of households when a child starts showing signs of fever and/or high temperature [3]. Herbal medicine comprises medicinal plants, minerals and organic matter extracted from a whole plant or parts of the plant like the roots, rhizome, leaf, pod, seed, bark, fruit or even the flower [2][5]. Herbal medicaments are found most commonly as water decoctions, ethanolic extracts, concentrates, tablets, capsules, tisanes, crude powders, ointments, salves, soft gels or tinctures [6]. Herbal medicinal powder is the granulated form of a medicinal herb used as an antidote for diseases [2] and have standards set by the World Health Organization with which they should be prepared in order for it to be fit for consumption [7]. Studies have identified bacteria (Escherichia coli, Bacillus cereus,etc.) and molds (Penicillium species, Fusarium species, and Aspergillus species) in herbal medicinal powders [8]. These medicaments are known to be highly susceptible to fungus like Aspergillus flavus which produces aflatoxin and other mycotoxins if not stored well [5]. Mycotoxins have the potential to be mutagenic, embryo toxic and carcinogenic [8]. These microscopic biochemical changes can only be detected by scientific observation yet this is not a common practice amongst herbal medicine manufacturers [9]. This study evaluates the microbial load and characterizes pathogens in three of the most patronized herbal powders in the Kumasi metropolitan area. | ||||||
|
Materials and Methods
| ||||||
|
Three different brands of herbal powder were collected from designated herbal shops in Kumasi, Ghana. The three samples were coded (A, B and C) for ethical reasons and kept sealed in their containers until time of analysis. The moisture content of the samples (2 g each) was determined using the method proposed by the international organization for standards (ISO), before microbiological analysis. In the microbial analysis, 1 g of test sample was weighed and dissolved in 9 ml sterile peptone water in a 15 mL Falcon tube to obtain 1-in-10 dilution. One milliliter was taken using a sterile pipette and was dissolved in 9 mL sterile peptone water in a 15 mL Falcon tube. The mixtures were diluted further 10-fold down to 10-6. Inoculum of 0.1 mL was taken from each of the serial dilutions using a sterile pipette and plated onto triplicate plate count agar (PCA) plates using a sterile L-shaped glass rod in spreading the inoculum. The PCA plates were then incubated at 35°C for 24 hours for growth to occur. The procedure was repeated for identification of molds and yeast using Sabouraud dextrose agar (SDA). Bacteria isolation and identification was done using MacConkey agar and xylose lysine deoxycholate agar. The plates were then incubated at 25°C (room temperature) for three days for growth to occur. Distinct colonies were sub-cultured onto corresponding to get pure cultures for the identification of the microbes. The identification of microbial colonies was done by observation of colony color, size, appearance and cell morphology. MacConkey agar distinguishes Gram-negative bacteria that can ferment the sugar lactose (lactose positive) from those that cannot (lactose negative). Xylose lysine deoxycholate (XLD) Agar is used in the isolation of Salmonella and Shigella species. Catalase and coagulase tests were performed to confirm the presence of pathogenic Staphylococcus aureus. | ||||||
|
Results | ||||||
|
Moisture content determination | ||||||
|
The moisture content of the three samples was in a range of 2.0–5.0%. According to the results recorded in Table 1, Sample A recorded 2.62% and has the lowest moisture content followed by Sample C which recorded 2.90% and finally B which recorded 4.70% (Table 1). | ||||||
|
Enumeration of microbes using plate count agar | ||||||
|
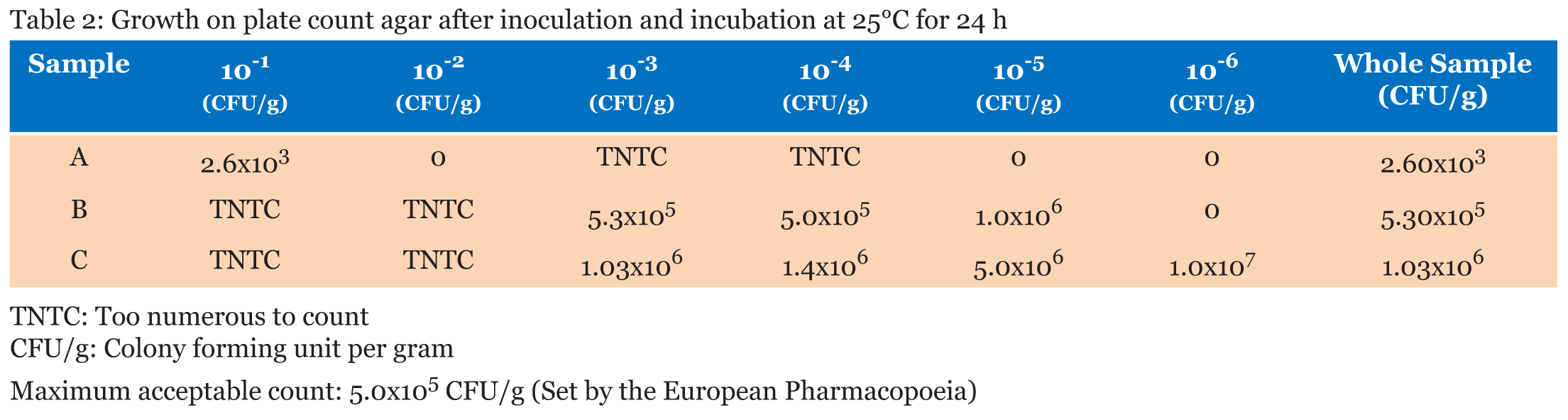
The aerobic plate counts ranged from a low of 2.6x103 CFU/g to a high of 1.03x106 CFU/g. The total number of microbes present in one gram of Sample A was recorded as 2.60x103 CFU/g. Sample B had a total of 5.30x105 CFU/g and Sample C recorded 1.03x106 CFU/g (Table 2). | ||||||
|
Identification of bacteria using MacConkey agar, xylose deoxycholate agar, mannitol salt agar, catalase and coagulase tests | ||||||
|
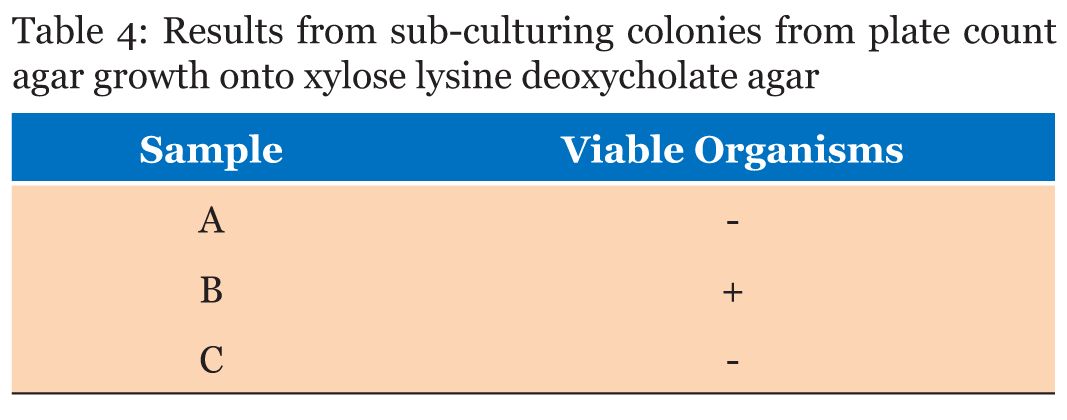
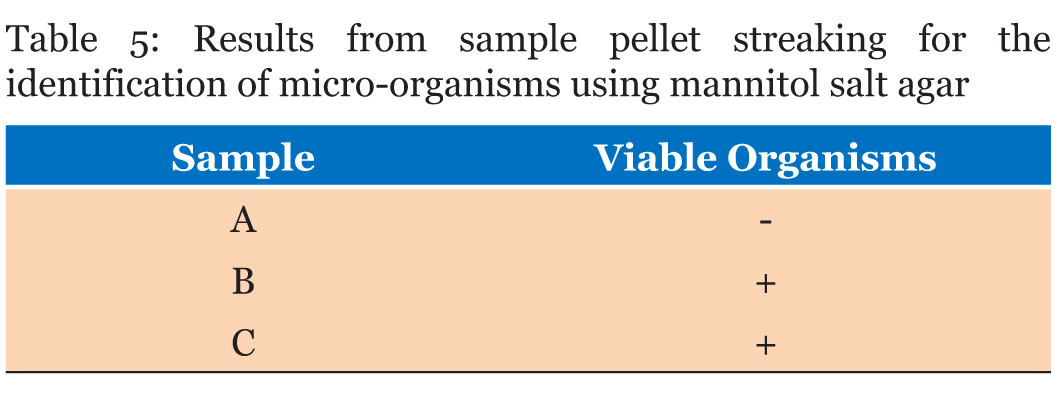
Significant growth in MacConkey agar was observed for plates with pellet streaks of Samples B and C whilst Sample A recorded no growth (Table 3). Samples A and C did not record any microbial growth on xylose deoxycholate (XLD) Agar after 48 hours of incubation and observation. Sample B recorded growth on the four plates the colonies were streaked onto (Table 4). The microbial analysis with mannitol salt agar depicted Sample A with no microbial growth after 48 hours of incubation. Samples B and C both recorded growth on their plates (Table 5). All the Samples gave positive results for the catalase test whilst only Sample C was positive for coagulase test (Table 6). | ||||||
|
Identification of yeasts and molds using Sabouraud dextrose agar | ||||||
|
The yeast and Mold counts ranged from a low of 8.0x102 CFU/g to a high of 5.8x104 CFU/g. Sample A recorded a total viable count of 8.0x102 CFU/g for yeasts and Molds in general. Sample B recorded 5.9x104 CFU/g and Sample C recorded 5.8x104 CFU/g (Table 7). | ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
In Ghana, around 65% of the population relies on herbal medicine alone to meet their basic healthcare needs [4]. However, medicinal plant materials normally carry a large number of microbes and the presence of these microbial contaminants has the potential to cause disease in humans when consumed. This study evaluates the microbial load and characterizes pathogens (if any) in three of the most patronized herbal powders in the Kumasi. | ||||||
| Moisture content determination | ||||||
|
The set standard by the European Pharmacopoeia Commission states that the moisture content of herbal powders should not exceed 7.5%. All three samples fell below this standard. The moisture content of a powdered product can provide an idea on its quality. It is an indication of its shelf-life; low moisture content is a requirement for a long storage life. The higher the moisture content in a powdered product, the more favorable the environment for micro-organisms to thrive. The results from this test imply that all three samples have acceptable moisture contents and would not support a favorable environment for micro-organisms to grow. The moisture content of the three samples was in a range of 2.0–5.0%. According to the results recorded in Table 1, Sample A recorded 2.62% and has the lowest moisture content followed by Sample C which recorded 2.90% and finally Sample B which recorded 4.70%. This could imply that Sample A would have the longest shelf life and Sample B will have the shortest shelf life. Since high moisture in powders are prone to earlier spoilage because of the increased activity of bacteria and fungi and the powder. Observing the way these three samples were packaged, it is no surprise that Sample B has the highest moisture content; the herbal powder was tied in a white polythene bag and placed inside a small container. On purchasing this drug, the lid was not tightly screwed onto the container leaving an avenue for moisture to enter it. Furthermore, Sample B had no batch number indicated on its packaging, it was not registered by Ghana’s food and drugs board and its manufacturing date was not indicated. All these bits of information are essential because it authenticates the drug. The only present information was the instructions on its use, the dosage, active ingredient and expiry date. Sample A came in an airtight container that had a seal that had to be broken in order to access the powder. No batch number was indicated on the package but it is registered by Ghana’s food and drugs board, had a manufacturing date and expiring date. Sample C also came in an airtight container that had a seal that had to be broken in order to access the powder. Batch number, manufacturing date and expiring date were present. It was registered by Ghana’s food and drugs board. These details are important for the manufacturer to display on the packaging, because it shows the current state of the product to the consumer. | ||||||
|
Enumeration of microbes using plate count agar | ||||||
|
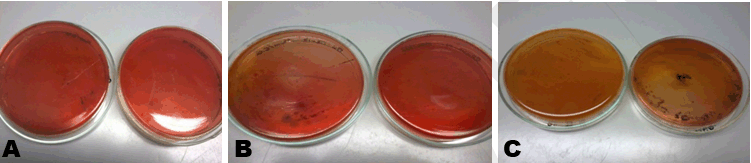
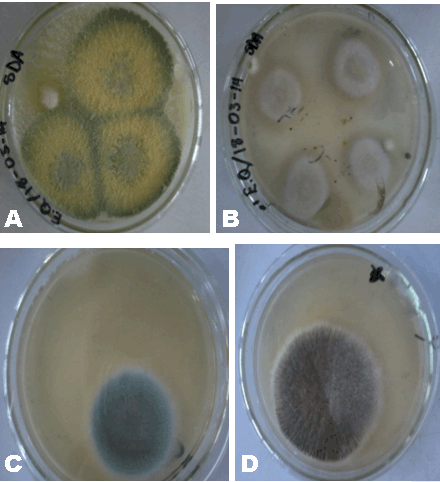
From Table 2, the aerobic plate counts ranged from a low of 2.6x103 CFU/g to a high of 1.03x106 CFU/g. The total number of microbes present in 1 g of Sample A was recorded as 2.60x103 CFU/g. Sample B had a total of 5.30x105 CFU/g and Sample C recorded 1.03x106 CFU/g. Plate count agar is used as a general indicator to assess the viable microbes in a Sample (Figure 1). The European Pharmacopoeia’s acceptance criterion for the total aerobic microbes count is 105 CFU/g and the maximum acceptable count is 5.0x105 CFU/g. In evaluating the microbiological quality of Sample A in reference to this standard, Sample A has a value below the maximum acceptable count, so A’s bacterial load can be said to be within acceptable range. Samples B and C, on the other hand, both have values above the maximum acceptable count which render their bacterial load unacceptable. Further analysis was performed on all the distinct colonies that formed on the media, to assess whether the microbes are pathogenic or not. | ||||||
|
Identification of bacteria using MacConkey agar, xylose deoxycholate agar, mannitol salt agar, catalase and coagulase tests | ||||||
|
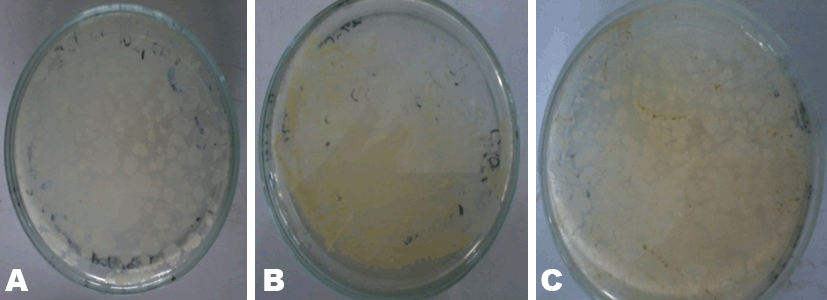
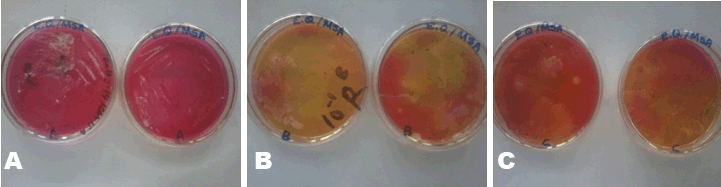
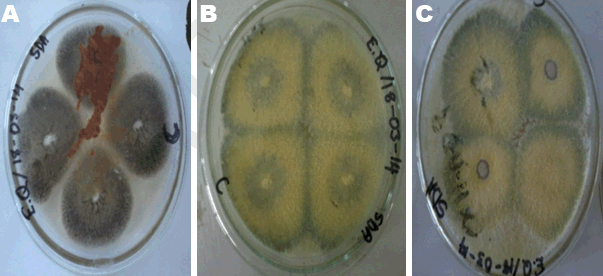
MacConkey agar was used to identify the presence of lactose fermenters such as Escherichia coli and Klebsiella and non-lactose fermenters in Samples A, B and C. MacConkey agar acts as a visual indicator, lactose fermenters produce acid by metabolizing the lactose present in the agar and as a result lowering the pH of the agar; this results in the appearance of reddish or pink colonies. The bile salts precipitate around the colony, causing the medium surrounding the colony to become hazy. From Table 3, only Sample A did not record any growth after 48 hours of incubation. Sample B recorded growth of pink colonies dispersed throughout the medium; this could be an indication of the presence of lactose fermenters. It was observed that there were a few white colonies formed as well. E. coli should be absent in 1 g of the Sample as set by the European Pharmacopeia Commission. The bacterial load for Sample B is unacceptable. Sorbitol-MacConkey agar can assist in the definite identification of the presence of enteropathogenic E. coli. Non-lactose fermenters cannot use lactose; instead they make use of peptone and forms ammonia as a result raising the pH of the agar. This leads to the formation of white colonies on the plate. From the results recorded for Sample C, the entire medium changed color to yellow (from red). It was observed that the colonies that grew on Sample C’s plates were white, circular and randomly dispersed; there were no pink colonies present (Figure 2). This is an indication of the presence of non-lactose fermenters such as Salmonella, Proteus species, Pseudomonas aeruginosa and Shigella; according to standards set by the European Pharmacopoeia, Salmonella should be absent in 25 g of a powdered Sample yet in this instance it is present in 1 g. Hence, it can be said that Sample C has an unacceptable bacterial load. Xylose deoxycholate (XLD) agar was used to analyze the distinct colonies that formed on the plate count agar. Xylose deoxycholate agar is used in the isolation of Salmonella and Shigella species from clinical samples and from food. Salmonella the intestine that can cause food poisoning, gastroenteritis, and typhoid fever. As a contaminant in products for human consumption it can cause Salmonellosis, a type of food poisoning usually characterized by gastrointestinal upset, diarrhea, fever, and occasionally death. Shigella is a dysentery causing bacterium and Shigellosis is a highly infectious form of dysentery caused by the Shigella bacterium. Samples A and C did not record any growth after 48 hours of incubation and observation. Sample B recorded growth on the fourth plates the colonies were streaked onto (Table 4). It was observed that a large portion of the media turned yellow whilst the sides remained red (Figure 3). Xylose deoxycholate agar has a pH of approximately 7.4, leaving it with a bright red appearance due to the indicator phenol red. Sugar fermentation lowers the pH and the phenol red indicator registers this by changing to yellow. Some of the colonies were reddish in color whilst majority of the other colonies were yellowish. On further incubation for another 24 hours, it was observed that some of the reddish colonies had gotten black centers. Salmonella, can ferment the sugar xylose to produce acid; Shigella colonies cannot do this and therefore remain red. Salmonellae metabolise thiosulfate to produce hydrogen sulfide, which leads to the formation of colonies with black centers and allows them to be differentiated from the similarly colored Shigella colonies [8]. The plates of Sample B show the presence of Salmonella, Shigella and coliforms; further analysis on isolating agar is required in order to name the species of the Salmonella and Shigella bacteria that are present in Sample B. The European Pharmacopoeia sets the acceptable criterion for Salmonella in a herbal medicinal powder that it should be absent in at least 25 g of a sample. Sample B is unacceptable in reference to this standard. Staphylococcus aureus is a bacterium that typically occurs in clusters resembling grapes normally inhabiting the skin and has the potential to cause disease by producing toxins responsible for boils, cellulitis, sty, impetigo, septicemia and food poisoning. Mannitol salt agar was used to isolate Staphylococcus aureus in the samples. Staphylococcus aureus produces yellow colonies with yellow zones, whereas other Staphylococci produce small pink colonies with no color change to the medium. Sample A did not record any growth after 48 hours of incubation. Samples B and C both recorded growth on their plates. The colonies were yellow, round and slimy (Figure 4). According to standards set by the European pharmacopoeia Staphylococcus aureus should be absent in 1 g of powdered sample. Samples B and C were unacceptable in respect to this standard. The catalase and coagulase test are confirmatory tests. The catalase test differentiates Staphylococci (catalase-positive) from Streptococci (catalase-negative). Coagulase test is used to differentiate Staphylococcus aureus from Staphylococci. Samples A, B and C all gave positive results for the catalase test indicating Staphylococci present in all of them. It was only Sample C that gave a positive for the coagulase test indicating the organisms picked from Sample C’s growth are Staphylococcus aureus (Table 6). | ||||||
|
Identification of yeasts and molds using Sabouraud dextrose agar | ||||||
|
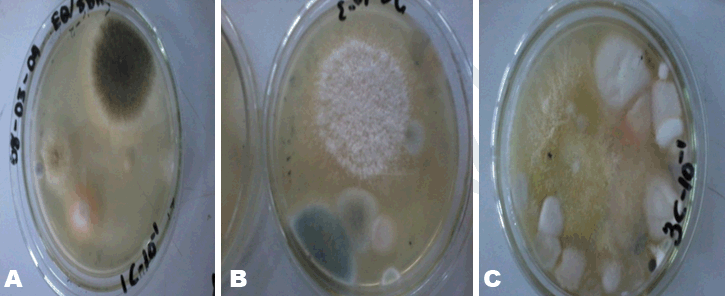
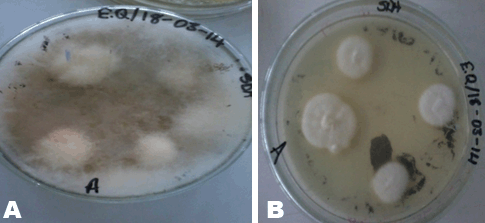
Herbal powders are susceptible to fungus like Aspergillus which produces aflatoxin and other mycotoxins. Mycotoxins produced by Aspergillus have the potential to be mutagenic, embryo toxic and carcinogenic [8]. For the microbial enumeration of molds and yeasts, Sabouraud dextrose agar was used. According to the set standard by the European Pharmacopoeia, the maximum acceptable count in 1 g of a powdered herbal medicinal sample is 5.0x104 CFU/g. Sample A’s count falls below 5.0x104 CFU/g indicating that Sample A has an acceptable fungal load. Sample B on the other hand, has a count above 5.0x104 CFU/g indicating that Sample B has an unacceptable fungal load. Sample C also has a count above 5.0x104 CFU/g (the 10-2 dilution had to be used instead of 10-1 because the number of organisms that grew on 10-1 plates fell below 30); this indicates that C has an unacceptable fungal load. On sub-culturing the distinct colonies that grew on to new sterile agar (Figure 5), pure colonies were formed and identified. Sample A’s colony was suspected to be Aureobasidium although the growth had not fully sporulated making the confirmation of its identity difficult (Figure 6). Sample B’s colonies included Trichoderma viride, Aureobasidium, Penicillium species and Aspergillus niger (Figure 7). Sample C’s colonies included Aspergillus niger and Trichoderma viride (Figure 8). Aspergillus niger is a common contaminant of food. Recent studies suggests some Aspergillus niger strains produce ochratoxin A, a potent mycotoxin. Trichoderma viride is less likely to cause human disease, it is a plant pathogen. Some Penicillium species produce mycotoxins such as patulin which are dangerous and deleterious to the immune system. Aureobasidium may cause allergic reactions when consumed over a long period of time [10]. | ||||||
|
Conclusion
| ||||||
|
The study demonstrated the presence of microbial contaminants in all three samples with that in Samples B and C exceeding the acceptable limits of microbial counts set by the European Pharmacopoeia. The presence of coliforms, Staphylococcus aureus, Salmonella, Aspergillus niger and Penicillium species constitute a health risk. Therefore, good pre-harvest and post-harvest practices together with hygienic manufacturing practices ought to be followed to minimize the level of microbial contamination. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
| Acknowledgements |
|
We would like to acknowledge staff of the Clinical Analysis Laboratory of the Department of Biochemistry and Biotechnology, KNUST for their assistance in this project. |
|
Author Contributions:
Eric Agyeman-Duah – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Esi Yaaba Quaidoo – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Felix Charles Mills-Robertson – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2017 Eric Agyeman-Duah et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|