|
|
Original Article
| ||||||
| Etiological agents isolated from stool samples of children under the age of five years in Windhoek, Namibia | ||||||
| Maria Amukoshi1, Innocent Maposa2, Sylvester Rodgers Moyo3, Munyaradzi Mukesi4 | ||||||
|
1Maria Amukosi, Graduate, Biomedical Sciences, Namibia University of Science and Technology, Namibia.
2Innocent Maposa, Lecturer, Mathematics and Statistics, Namibia University of Science and Technology, Namibia. 3Sylvester Rodgers Moyo, Professor, Biomedical Sciences, Namibia University of Science and Technology, Namibia. 4Munyaradzi Mukesi, Lecturer, Biomedical Sciences, Namibia University of Science and Technology, Namibia. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Amukoshi M, Maposa I, Moyo SR, Mukesi M. Etiological agents isolated from stool samples of children under the age of five years in Windhoek, Namibia. Edorium J Microbiol 2017;3:1–9. |
|
Abstract
|
|
Aims:
Diarrheal diseases constitute a major health problem globally, especially in lower income groups in developing countries. The majority of deaths from diarrhea occur in children under the age of five years. The aetiology of diarrhea is huge and comprises different bacteria, parasites, viruses and other factors like malnutrition, micronutrient deficiencies, milk and food intolerances, diseases of the bowel or prior antibiotic therapy.
Methods: This study evaluated intestinal parasites and bacteria isolated from stool samples of children under the age of five years in Windhoek, Namibia, as well as determine the antimicrobial susceptibility patterns of the isolated intestinal bacteria. This was a retrospective review of intestinal parasitic and bacterial pathogens isolated from stool samples of children under the age of five analyzed at the Namibia Institute of Pathology (NIP), Windhoek Central laboratory during the period 2012 to 2014. A total of 1392 stool sample records of children under the age of five years were analyzed for the presence of intestinal parasites and bacteria, and the antimicrobial susceptibility patterns of the isolated bacteria were determined. Results: Pathogens were isolated from 236 (17%) of the samples that were analyzed. Salmonella species were the most isolated enteropathogen 36 (2.6%) followed by Giardia lamblia with 26 (1.9%). The ≤12 months age group had the highest frequency of bacterial isolates 35 (45.5%) while the highest frequency for parasites 11 (31.4%) was from the >13–≤24 months age group. Majority of the bacterial isolates were resistant to amoxicillin and highly susceptible to ciprofloxacin and ofloxacin. Conclusion: Salmonella species were the most common intestinal pathogens isolated from the stool samples of children under the age of five while Giardia lamblia was the common intestinal parasite that affects younger children. | |
|
Keywords:
Antimicrobial, Bacteria, Children, Giardia lamblia, Parasites, Salmonella, Stool
| |
|
Introduction
| ||||||
|
Diarrheal diseases are a major public health concern globally, with over two million deaths occurring each year, and affecting mostly children under five years of age [1]. Diarrhea is usually a symptom of an infection of the intestinal tract, which can have aetiologies including bacteria, viruses or parasites. The majority of deaths from diarrhea are among children under the age of five, living in low and middle income countries. It is estimated that a child dies every 15 seconds from diarrhea especially in areas where there is poor sanitation and contaminated water supplies [2]. Lacks of access to safe water sources, inadequate sanitation and poor hygiene have contributed largely to diarrheal infections and deaths worldwide. Children living with HIV/AIDS are mostly at risk, with about 36–50% with persistent diarrhea at initial presentation at the hospital [3]. Even though diarrhea mortality and morbidity has decreased since the 1990s, the disease is still a huge burden especially in developing countries [4] . However, with the introduction of some vaccines such as the rotavirus vaccine that was introduced by the World Health Organization (WHO) in 2006, there has been a decline in the mortality and morbidity attributed to rotavirus diarrhea and diarrhea caused by other factors, in countries that have started using the vaccine [5]. It is widely recognized that exposure to diarrhea pathogens in developing countries is associated with the age of the child, poor quality and quantity of water, availability of toilet facilities, housing conditions, level of education, household economic status, place of residence, feeding practices, and the general hygiene practices. Socioeconomic factors may directly and indirectly affect the environmental, behavioral, nutritional, and demographic risk factors, with the exception of age and sex [2]. The increase in antibiotic resistance among enteric pathogens in developing countries also becomes an important issue of concern. Overuse and misuse of antibiotics in the treatment of diarrhea could lead to an increase of antibiotic resistance. In developing countries where laboratory diagnosis is very poor, physicians are usually forced to diagnose patients based on symptoms and they prescribe broad spectrum antibiotics which could lead to emergence of drug resistant strains [2]. | ||||||
|
Materials and Methods
| ||||||
|
Data for this study was retrieved from NIP's laboratory information system. Study population A total of 1392 records which met the criteria were reviewed. Sampling procedure This study used stool sample results reported for macroscopic examination, microscopic examination using saline wet preparation techniques (for detection of protozoan trophozoites, cysts and eggs or larvae), formal ether concentration and stool culture. Culture was done on xylose lysine deoxycholate (XLD) agar, deoxycholate citrate agar (DCA), MacConkey with crystal violet and Selenite F broth. Enteric bacteria isolated were identified using biochemical tests and API® 10 S (bioMérieux) and processed according to the manufacturer's instructions. Polyvalent agglutinating sera were used for serotyping organisms. Antimicrobial susceptibility of the bacterial strains was tested using the Kirby-Bauer disk diffusion method on Mueller-Hinton agar (MHA) according to the Clinical Laboratory Standards Institute (CLSI) guidelines (2012, 2013 and 2014). Candida species were identified using either the germ tube method or CHROMagar™. Yeasts were classified as Candida albicans, Candida tropicalis or yeasts not Candida albicans. Data analysis Ethical consideration | ||||||
|
Results | ||||||
|
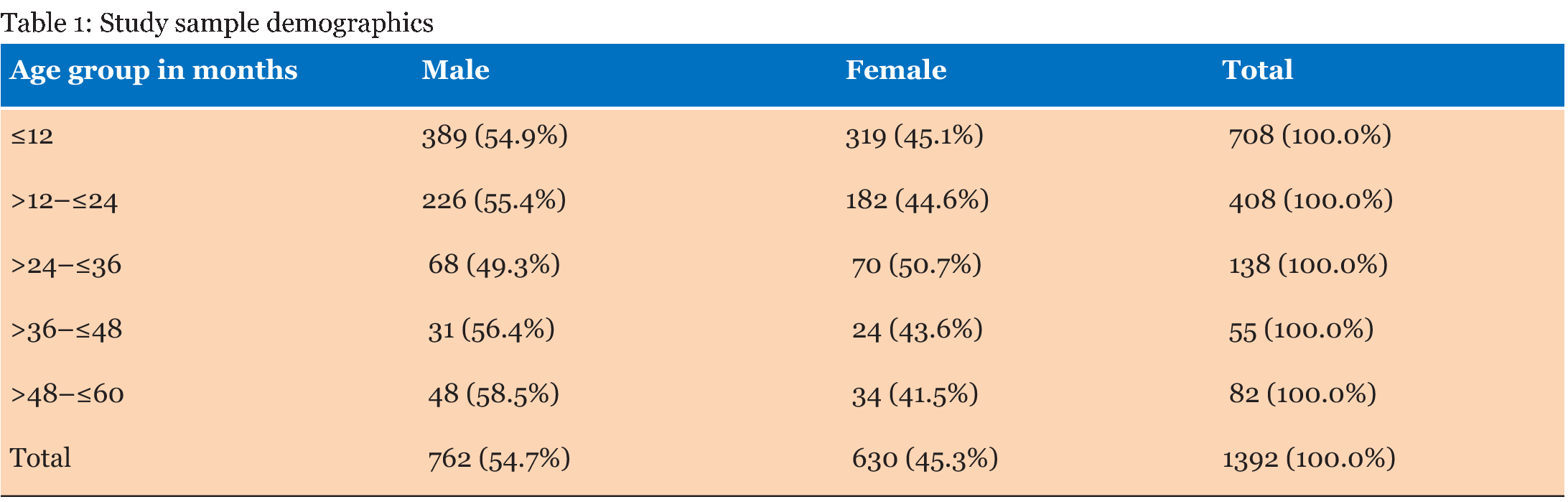
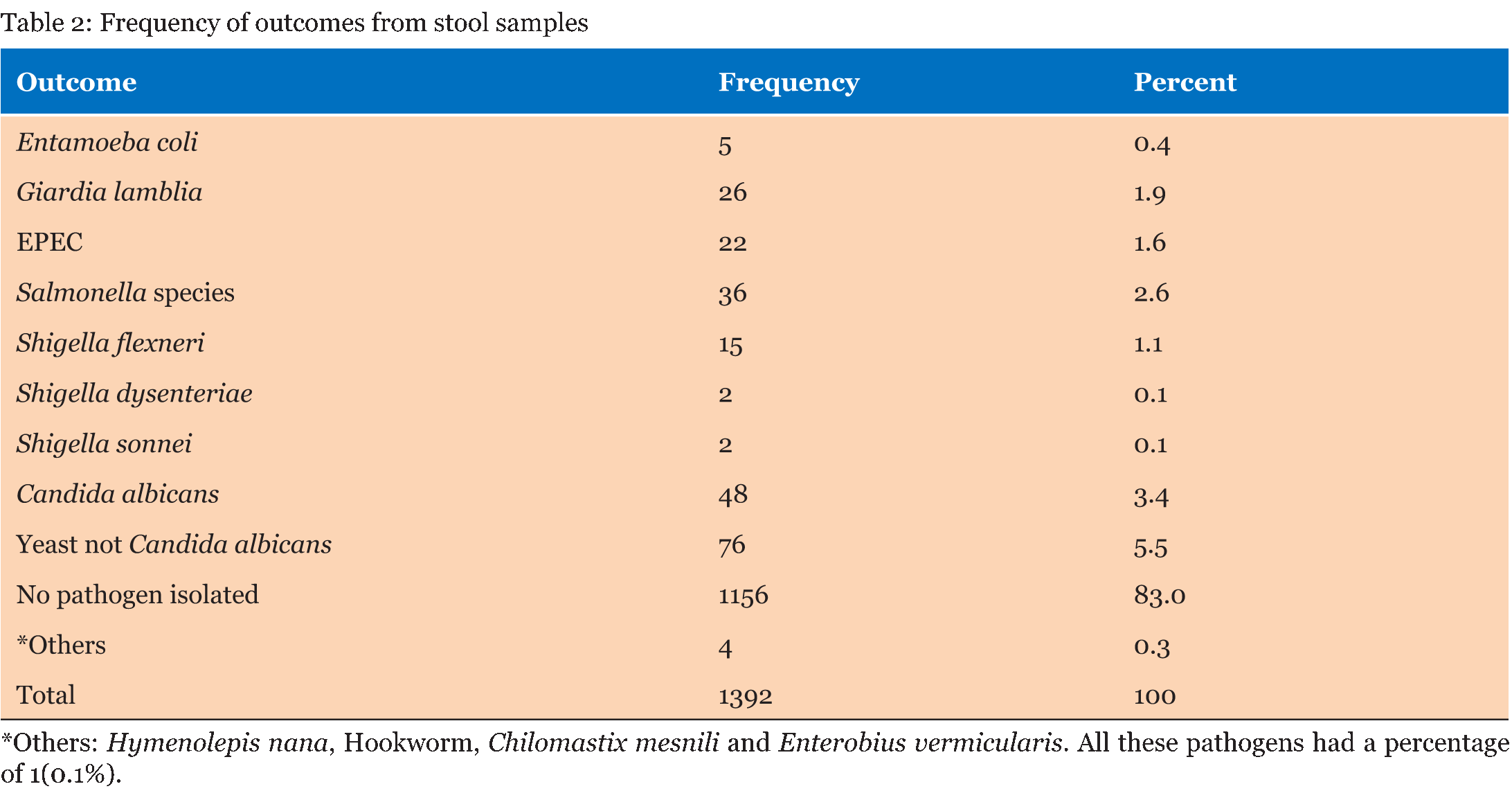
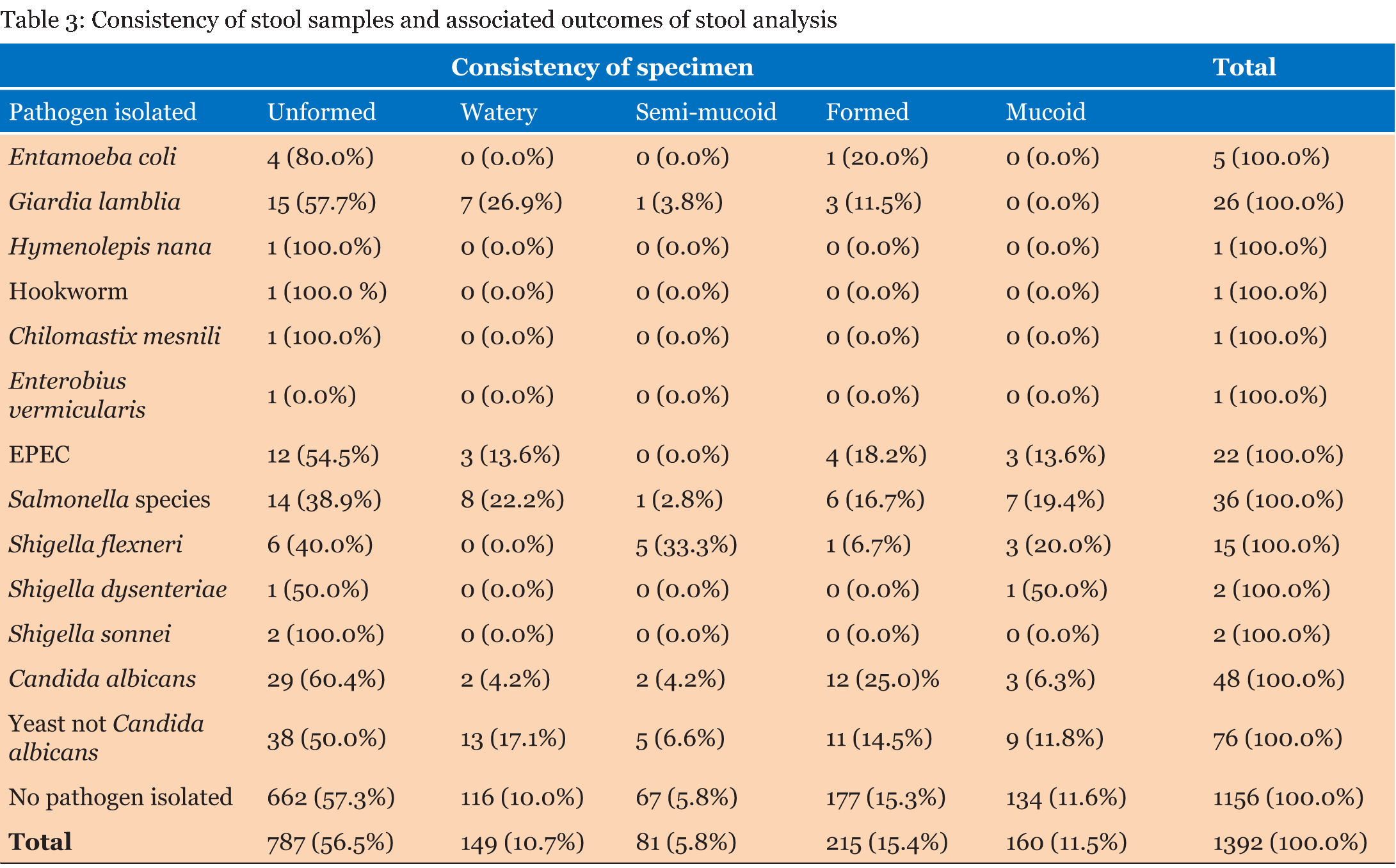
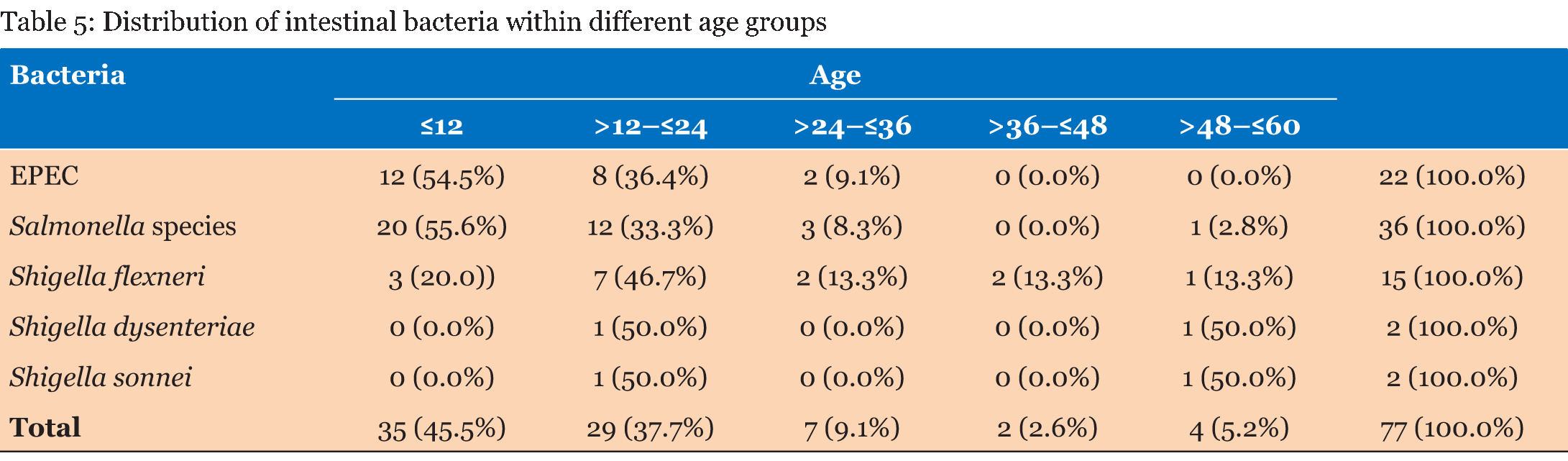
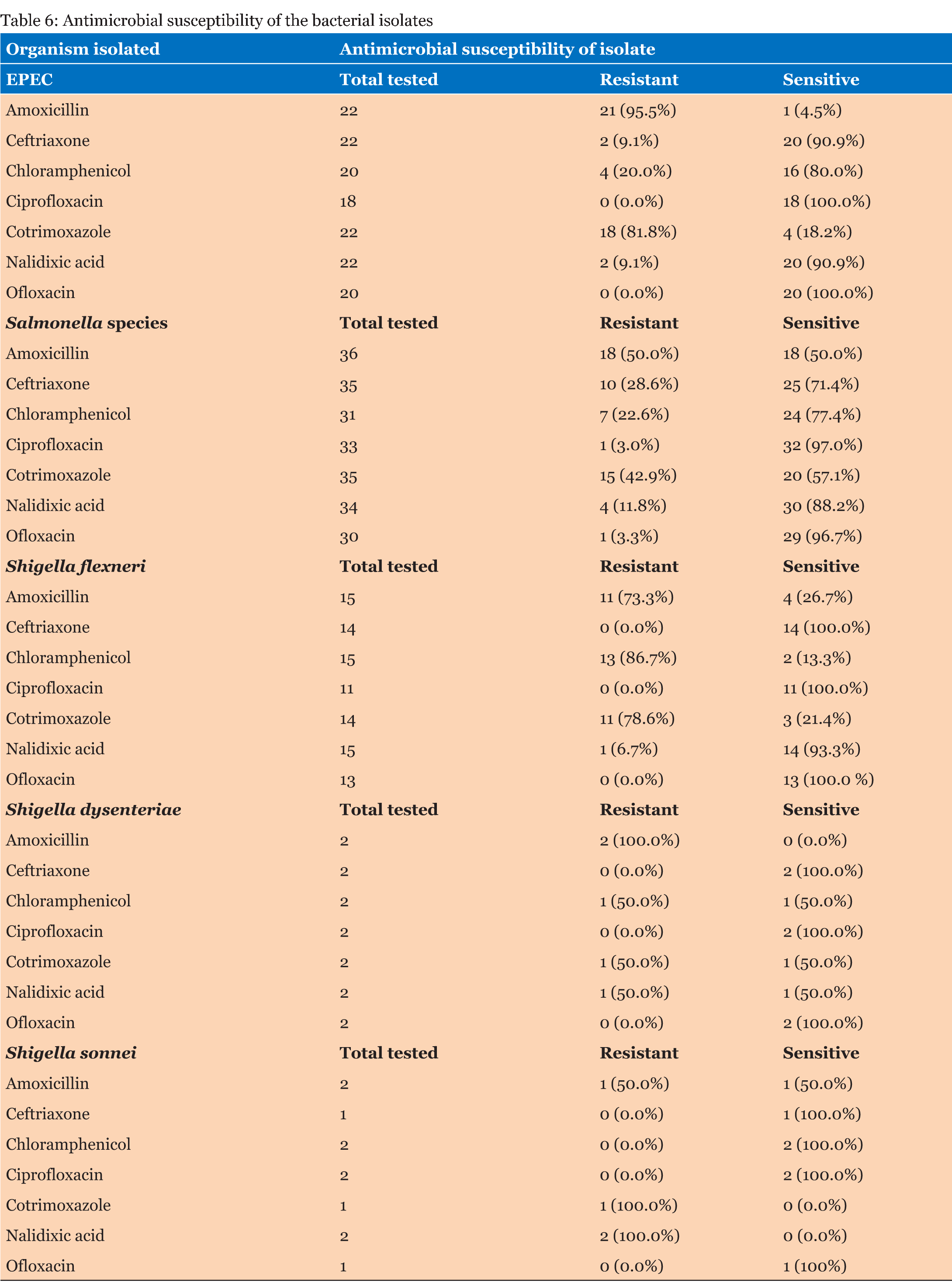
A total of 1392 laboratory reports of children under the age of five years were analyzed for the presence of enteropathogens. Out of the 1392 reports analyzed, 762 (54.7%) were for male children and 630 (45.3%) were for female children. Table 1 gives that the majority of samples (708) were from children ≤12 months and the least number of samples were from children aged >36–≤48 months. There were more male children (54.7%) than females. The frequency of outcomes from stool samples is given in Table 2. Pathogens were found from 236 (17.0%) of the samples that were analyzed. Bacteria of the Salmonella species were the most isolated enteric bacterial pathogens, Giardia lamblia was the most common parasite while yeasts not Candida albicans were the most common fungi isolated from the stool samples. Table 3 gives that most of the analyzed stool samples 787 (56.5%) were unformed. Watery stools were 149 (10.7%). No pathogens were isolated from about 90% of the watery samples. Most of the enteric pathogens isolated were from unformed stools. Formed stools had more yeasts and bacterial pathogens than parasites. The highest number of the parasites 11 (31.4%) were found from the >12–≤24 months age group. Giardia lamblia was the most common parasite in this age group, with a frequency of 10 (38.5%). No parasites were found in the =12 months age group as given in Table 4. Table 5 gives that most bacterial isolates 35 (45.5%) were isolated from the ≤12 months age group and the least number of bacterial isolates was from >36–≤48 months age group. Enteropathogenic Escherichia coli (EPEC) was only isolated from children between the ages of =36 months. Salmonella species were the most common enteric bacteria 20 (55.6%) isolated in ≤12 months age group. Table 6 gives the antimicrobial susceptibility of bacterial isolates. Most bacterial isolates were resistant to amoxicillin. EPEC showed a high degree of resistance to amoxicillin 21 (95.5%) and Shigella flexneri showed 11(73.3%) resistance to the same antibiotic. High resistance to cotrimoxazole was observed with EPEC 18 (81.8%), and Shigella flexneri 11(78.6%). Salmonella species were resistant to amoxicillin (50.0%) and to cotrimoxazole (42.9%). Shigella dysenteriae and Shigella sonnei isolates were too few to provide a meaningful antimicrobial sensitivity analysis. | ||||||
| ||||||
| ||||||
| ||||||
| ||||||
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
This study investigated the occurrence of enteropathogens in both diarrheal and non-diarrheal stools samples that were obtained from children under the age of five years in Windhoek, Namibia during the period 2012 to 2014. The prevalence of these pathogens was lower (17%) compared to the results of other studies. In a study done in Ouagadougou, Burkina Faso, the prevalence of diarrheal pathogens was higher in children under five years, accounting for about 64% of the cases [1]. In a similar study done in Jimma, Southwest Ethiopia [6], the prevalence of intestinal pathogens was reported in children under five years to be 49.6%. This difference may be due to the fact that not all bacterial agents e.g., Campylobacter and Yersinia and viruses e.g. Rotavirus were tested in our study which led to the low number of pathogens. This was a major limitation in this study. Furthermore, variations in reported frequency may be due to the diagnostic tools used, rather than the actual incidence of each pathogen [7]. For example, the study done in Ouagadougou, Burkina Faso used a combination of conventional and molecular diagnostic methods to detect and identify enteropathogens [1], while our study only used conventional microbiology techniques. Furthermore, variations may be due to differences in geographic setting of the study and also the season in which the study was conducted and these are factors with a huge impact especially on diarrhea [7]. Thirty-five (2.5%) stool samples analyzed in this study were positive for intestinal parasites. This figure is relatively lower compared to a study done in Ashanti Akim, Northern Ghana, where the prevalence of intestinal parasites in children under the age of five years was 10.9% [8]. In a study done in an informal settlement in Nairobi, Kenya the prevalence of intestinal parasites was 25.6% [9] and in Addis Ababa, Ethiopia (34%) [10]. These differences may be due to the difference in geographical locations, as parasites are more common in tropics and subtropics. Although Namibia is in the subtropics it has low humidity which makes it difficult for parasites to thrive [11]. Giardia lamblia was the most common parasite 26 (1.9%). This result is similar to that obtained in Dar es Salaam, Tanzania where the frequency of Giardia was also 1.9% [12]. The result was also close to the prevalence obtained in Maniça District, Southern Mozambique with a prevalence of 2.5% [13]. However, in other parts of Africa, the prevalence was much higher. In Nairobi, Kenya, the prevalence of Giardia was 4.6% [9], and in Jimma southwest Ethiopia the prevalence in children under the age of five years was 21.7% [6]. Parasitic diarrhea in children due to Giardia lamblia infection, is particularly common in areas where fresh vegetables and drinking water sources are contaminated with sewage, and food purchased from street vendors hence water and sanitation have a huge role to play [8]. Of the 1392 stool samples that were tested in this study 77 (5.5%) of them were positive for intestinal bacterial pathogens. This figure is lower compared to a study done in Addis Ababa, Ethiopia where the prevalence was 41% [10]. In Ouagadougou, Burkina Faso, the prevalence of intestinal bacteria was 40% [1] and in Dar es Salaam, Tanzania it was 33.3% [12]. In the current study, Salmonella species were the most common 36 (2.6%) bacterial isolates. This prevalence was consistent with that of studies done in Hawassa, South Ethiopia and in Dar es Salaam, Tanzania, both with a prevalence of 2.5% [12] [14]. The prevalence of Shigella species 19 (1.3%) from our study was consistent with that of a study done in Jimma, Southwest Ethiopia where the prevalence of Shigella species was 2.3% [15]. In the current study, EPEC was isolated from 22 (1.6 %) stool samples. This prevalence is lower compared to studies done in Ouagadougou, Burkina Faso where the prevalence was 24.0% [1] and in Addis Ababa, Ethiopia (24.1%) [10]. In Cairo, Egypt, the prevalence of EPEC was 5.2% [16]. These differences in findings may be due to the fact that in our study, only stool samples of children under the age of two years were tested for EPECs. Differences in the method of identifying E. coli species may also have played a big role. Some studies have used PCR to characterize diarrheagenic E. coli (DEC) into pathotypes based on virulence genes, while in our study, only culture, biochemical reactions and serotyping where used to identify EPEC. PCR has been proven to have a high sensitivity and specificity compared to `most techniques that have been used to identify DEC [17]. In this study, 8 (22.2%) Salmonella species were isolated from watery stool samples. This result is relatively high compared to a study done in Butajira, central Ethiopia where 10% of Salmonella isolates were recovered from watery samples [18]. In another study done in Harar, Eastern Ethiopia, no Salmonella was isolated from watery stools [19]. In the same two studies, isolates of Salmonella were also obtained from mucoid stools, which is consistent with our findings. No Shigella was isolated from watery stool samples in the current study, while with the study done in Butajira, central Ethiopia, about 17.6% of Shigella species were isolated from watery stools [18]. In the present study, Giardia lamblia was frequently found from unformed stools 15 (57.7%) and from watery stools 7 (26.9%), which is comparable to a study done in Ashanti Akim North, Ghana where 86.0% of Giardia was isolated from semi-formed stool samples and 12.9% isolated from loose stool samples [8]. No parasites were found in the =12 months age group in the current study. This result is consistent with the results of a study done in Jimma southwest Ethiopia, where no parasites were found from the same age group [6]. The results of a study done in Nairobi, Kenya also indicated that Giardia was not common in children under the age of 12 months and only 1.3% was found in that study [9]. The low prevalence of parasites in children under the age of 12 months may be due to the fact that these children are inactive and rarely come into contact with soil, contaminated water or domestic animals which are a source of most parasites. Giardia is more common in active children who normally play with soil which could be contaminated with parasites and since they rarely employ good sanitary behavior, they do not wash their hands with soap after playing or after using the toilet [8] [9]. Children of pre-school age who are usually in child care settings such as day care centers are more at risk. In this study, more than 60% of the bacterial enteropathogens were found from children in the =24 month age group. This is comparable to the results of a study done in Ouagadougou, Burkina Faso were more than 60% of intestinal bacteria were isolated from children under the age of 24 months [1]. In the same study, DEC was mostly isolated in children under 12 months of age (51%) which is consistent with the results of our study 12 (54.5%) in the same age group. In Southern Mozambique, the prevalence of DEC was as high as 61% in children younger than one year [13]. However, these results were much higher compared to the 7.5% that was obtained in children under the age of 12 months in a study done in Cairo, Egypt [16] . In the same study, most DEC isolates (17.8%) were from children between 25–36 months. This variation in the occurrence between different age groups may be due to the fact that young children are more susceptible to enteropathogens, but as they grow they acquire some resistance to enteric pathogens. Antibiotic susceptibility data from the current study show that E. coli is highly resistant 21 (95.5%) to amoxicillin. This is consistent with the results of a study carried out in Maradi, Niger where the resistance was about 88.9% [20] and in Abuja, Nigeria where the resistance of E. coli to amoxicillin was 73% [21]. Resistance to most antibiotics is found in DEC isolated from children with diarrhea in developing countries where overuse and misuse of antibiotics is common [22]. Fecal E. coli is also regarded as an important indicator of the spread of acquired antibiotic resistance genes in the communities [23]. Resistance of E. coli isolates in children has developed over the years and this is a problem in both the community and hospital settings [22]. In our study, E. coli isolates were very susceptible (100.0%) to ciprofloxacin and ofloxacin, which is consistent with the results of a study done in Khartoum, Sudan where the susceptibility to ofloxacin was 93% [24]. Salmonella species isolated from this study had considerable resistance to amoxicillin (50.0%) and to cotrimoxazole (42.9%). Resistance of Salmonella to these antibiotics has also been noted in Harar, Eastern Ethiopia [19] where the resistance was 100%, in Addis Ababa, Ethiopia (80.0%) [10] and Jimma, southwest Ethiopia where resistance to amoxicillin was 100% [6]. As with other bacterial isolates Salmonella was highly susceptible to ciprofloxacin (97.0%) and ofloxacin (96.7%). Shigella flexneri, which was the most isolated Shigella species was resistant to chloramphenicol (86.7%), cotrimoxazole (78.6%) and amoxicillin (73.3%). The high amoxicillin resistance rates are comparable to those of a study done in Hawassa, South Ethiopia where there was absolute resistance [14]. In the same study, resistance to chloramphenicol was low (9.1%) compared to our resistance rate of 86.7% to chloramphenicol. The development of high resistance of Shigella flexneri was also reported in Maradi, Niger (71.8%) [20] and in Madagascar (92%) [25]. These results are consistent with those of studies done in Mwanza, Tanzania where the resistance to cotrimoxazole was 97% [26], and in Madagascar (77%) [25]. Non availability of data on Rotavirus and Campylobacter was a major limitation in our study. This study was based on secondary data and a distinction could not be made between acute and persistent diarrhea. A number of bacterial isolates were also too few to offer statistically significant analyses of susceptibility patterns. The results of this study are very useful in the management of diarrhea in children under the age of five years, which is the age group that needs more attention when it comes to health issues. Knowledge of the pathogens associated with diarrhea is very important for clinicians in determining interventions to manage the factors causing diarrhea such as preventive vaccines, therapeutic approaches and determining appropriate environmental sanitation programmes in communities. | ||||||
|
Conclusion
| ||||||
|
Namibia has a low prevalence of intestinal pathogens compared to other African countries. Most of the enteric pathogens isolated were bacterial pathogens. Salmonella species were the most common bacteria while Giardia lamblia was the most common parasite found. Occurrence of bacterial enteropathogens was also noted to occur mostly in children =24 months of age. The results of our study also indicated that bacterial isolates have developed resistance to most commonly used antibiotics. Comprehensive studies need to be done in order to understand the whole aetiology of diarrhea in children, in order to improve management and treatment. | ||||||
|
Acknowledgements
| ||||||
|
We would like to thank the Department of Biomedical Sciences, Namibia Institute of Pathology and Ministry of Health and Social Services, Namibia for their support. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Maria Amukoshi – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Munyaradzi Mukesi – Substantial contributions to conception and design, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Innocent Maposa – Acquisition of data, Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Sylvester Rodgers Moyo – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2017 Maria Amukoshi et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|